AI tools such as ProteinMPNN are now widely used to design new proteins—or to redesign existing ones for greater stability. In many studies, the redesigned proteins end up more stable than the original “template” protein. But a basic question has been hard to answer: how does AI actually make a protein more stable?
In our earlier work (ACS Synthetic Biology, 2023), we showed that ProteinMPNN can redesign ubiquitin variants with improved binding affinity and striking stability. After that paper, we realized just how extreme the stability was: the variants’ melting temperatures (Tm) were beyond our instrument limit of 120 °C. They also resisted unfolding under conditions that normally destroy proteins—strong acidity at high temperature, and even 8 M urea, where the variants still remained folded and native-like.
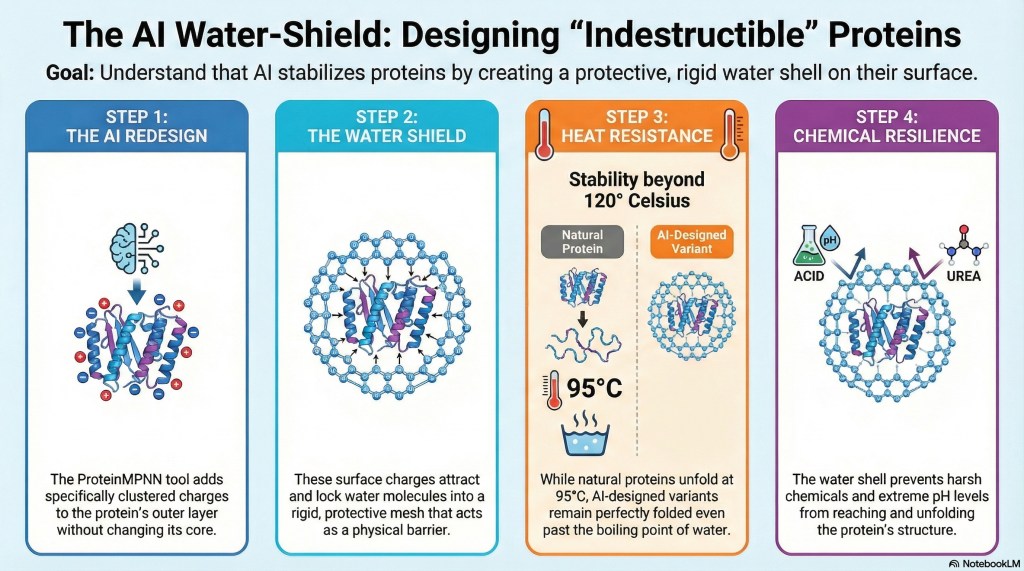
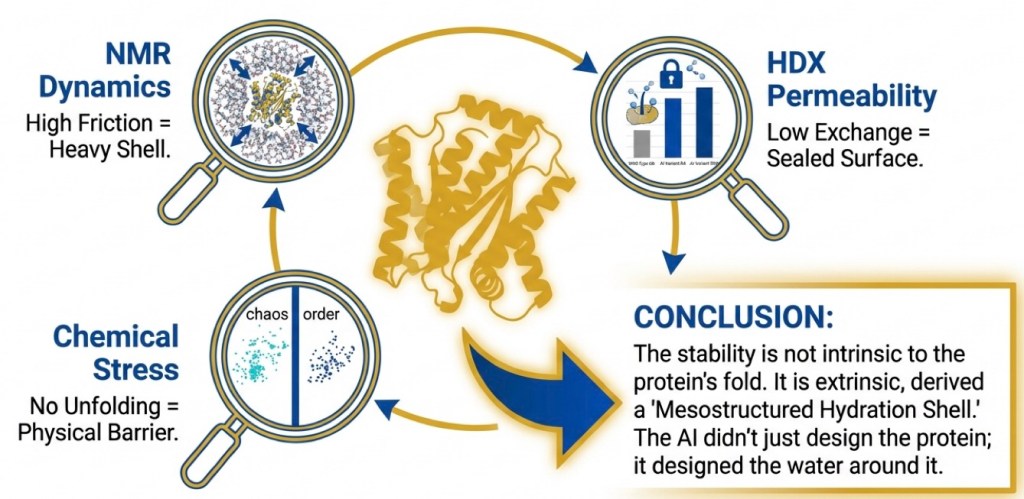
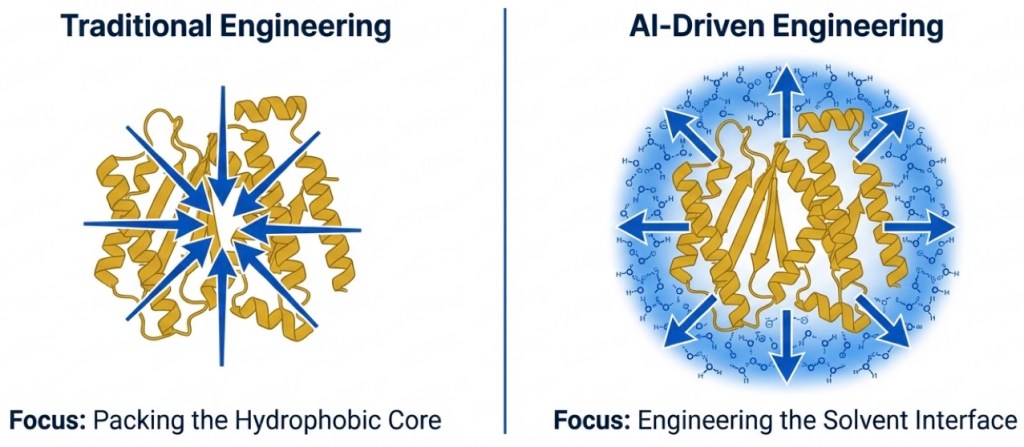
Our more recent study in the Journal of the American Chemical Society helps open this “black box.” Using solution NMR spectroscopy, biophysical measurements, and molecular dynamics simulations, we found a clear mechanism: a thicker, more persistent hydration shell forms around the AI-designed proteins, and this water “coat” helps protect them from denaturation. Rather than tightening the hydrophobic core, ProteinMPNN largely preserves it while reshaping the protein’s surface—creating electrostatic patches that attract and organize water molecules. Simulations show the designed ubiquitin variants retain more water in their primary and secondary hydration shells than wild-type ubiquitin. In high-temperature simulations, the variants stay stable while ubiquitin more readily unfolds and misfolds.
This is especially notable because ubiquitin is already a very stable protein (Tm ≈ 96 °C), and further improving its stability is typically difficult. In fact, many ubiquitin variants generated by experimental display methods show reduced thermal stability. In contrast, ProteinMPNN produced variants with Tm > 120 °C with surprising ease.
And this effect is not limited to ubiquitin. As a second test case, we redesigned another ubiquitin-fold protein domain: the C-terminal domain of ISG15 (ISG15-CTD). ISG15-CTD normally has a much lower stability (Tm ~55 °C), but ProteinMPNN redesign boosted it dramatically—again reaching ~110–120 °C. NMR and biophysical data indicate that these ISG15-CTD variants follow the same pattern as the ubiquitin variants, consistent with the hydration-shell stabilization mechanism.
This insight could reshape how we think about protein design. Instead of treating stability as a problem solved mainly by “better core packing,” our work suggests a complementary design principle: engineer the protein surface to recruit and organize a protective hydration shell. In practice, this means AI can be used not only to redesign sequences for function, but also to tune how proteins interact with water—a key factor in real-world robustness. The implications are broad for biotechnology and biopharma: more heat- and chemical-resistant protein reagents, longer shelf life and easier shipping/storage, improved manufacturability, and potentially more durable protein therapeutics and enzyme catalysts that tolerate harsh formulation and process conditions. In other words, designing proteins may increasingly mean designing the protein–water system as a whole, enabling scalable, reliable protein-based applications across research, diagnostics, and medicine.
The paper, “Mesostructured Water Enhances Stability of ProteinMPNN-Designed Ubiquitin-Fold Proteins,” was authored by the Wu lab members, and colleagues (Academia Sinica, UC Riverside, Osaka University). The work was supported by Academia Sinica Grand Challenge Seed Grant (AS-GCS-113-L05), Innovative AI Applications in Humanities and Scientific Research (I-AI-A) Projects (AS-IAIA-114-L02), and National Science and Technology Council (NSTC 114-2113-M-001-019) (Dr. Wu). First author Lu-Yi Chen was supported by the NSTC Graduate Research PhD Fellowship. This is an open access article (CC-BY-4.0)
A slide and a video generated by NotebookLM are providing here to summarize our research outcome.